DePuy Orthopaedics, a Johnson & Johnson subsidiary, specializes in orthopedic surgical and joint replacement devices with over 200 individual products to its name. In close to 100 years, DePuy has developed hundreds of devices that have been successful, but three devices in the hip replacement category have shown a higher rate of failure. These devices include the ASR Hip Resurfacing System, the ASR XL Acetabular System, and the Pinnacle Hip Replacement System. DePuy and Johnson & Johnson are facing lawsuits brought by patients who’ve experienced problems with these devices and have suffered severe and permanent injuries.
How the DePuy Hip Devices Work
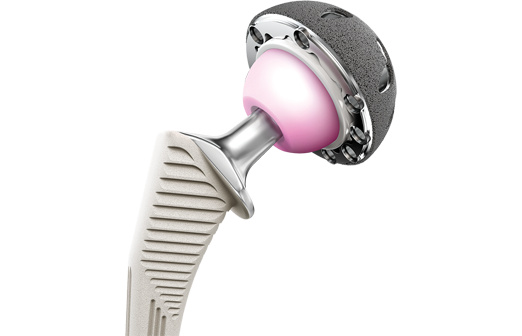
The hip joint is comprised of a ball and a socket where the ball fits into the socket. The “ball” is the femoral head at the top of the thighbone known as the femur and the socket portion is known as the acetabulum. As the leg moves, the femur rolls inside the curved surface of the acetabulum. A hip replacement is designed to replicate the action of the hip joint. It has three parts; a ball, which replaces the femur, a stem, which is inserted into the femur, and a cup, which fits into the pelvis and replaces the acetabulum. Hip implants are often explored when an individual experiences a broken hip, serious hip fractures, or from osteoarthritis, a degenerative condition that targets joints, especially the hips.
Like many other hip implant devices, the faulty DePuy products have been constructed with metal-on-metal (MOM) construction. This means that the ball and socket of the hip implant are made of metal. Manufacturers of MOM devices designed the implants to be durable enough to last longer than alternative implant systems made of polyethylene or ceramic. They were meant to increase mobility for physically active patients and younger replacements who would want to return to work or engage in athletic activities.
With an ASR Hip, an acetabular cup replaces the acetabulum. The DePuy ASR Hip Resurfacing System, on the other hand, consists of two components; a cap that’s fixed over the natural femoral head and a metal cup that replaces the acetabulum. The ASR XL total hip replacement has there components, which are aligned to restore a more natural movement. With this device, the femoral stem is inserted inside the femur and then the ball (femoral head) connects to the femoral stem, which then fits inside the acetabulum.
Both the ASR XL Acetabular System and ASR Hip Replacement are alternatives to full hip replacement surgery, which involves the removal of the hip and replacing it in its entirety. The ASR XL Acetabular System involves placing a metal cap on the femoral head thereby conserving it. Conversely, the ASR Hip Replacement is a form of hemiarthroplasty, which involves replacing parts of the hip with implants as opposed to removing it.
DePuy’s Hip Replacement Devices were placed in the market in 2003 and wildly marketed as advanced metal-on-metal implants. More than 93,000 DePuy hip implant devices were used worldwide, accounting for about 23 percent of the total hip replacement market.
The Problem with DePuy Hip Replacements
After your hip replacement, you likely looked forward to pain-free, restored mobility, perhaps for the first time in a long time. Unfortunately, you may end up experiencing disability and increased pain after the implant. The reason for this may be a defective DePuy hip implant. While the MOM design was meant to be an advance in hip replacement technology and to significantly improve mobility, it has instead caused unexpectedly high failure, particularly due to the metals grating against each other, releasing small amounts of metal. Many DePuy implants failed within a year or two after being implanted, proving to be far less serviceable than conventional devices.
The moving parts of the ASR and Pinnacle implants are made of a cobalt-chromium alloy, which is a more durable material than ceramic or plastic. But these metal-on-metal implants release metal ions as the surface of the cup and the ball rub together. The tiny chromium and cobalt particles are released into the surrounding tissues causing severe pain and inflammation. What’s more, the particles get into the bloodstream and can potentially damage organs such as the heart and brain.
Side Effects and Symptoms Linked to DePuy Hip Implants
Asignificant number of patients fitted with DePuy hip replacement devices have reported serious symptoms and side effects, including:
- Loosening or instability in the hip joint due to the implant’s failure to properly attach to the bone
- Fractures from the bone around the implant breaking
- Dislocation where the two parts of the hip implant become misaligned
- Grinding, clanging, or clicking sound coming from the hip area
- Pain in the hip, leg, back, and groin
- Fluid build-up, inflammation, and swelling at or near the hip joint
- Limping and difficulty walking or moving the hip properly
- Nerve and bone damage that can be permanent
- Muscle atrophy due to immobility
- Elevated levels of chromium, cobalt and other metals in the bloodstream
- Bone dissolution from metal poisoning (osteolysis)
- Bone and other tissue death due to metal poisoning (necrosis)
- Formation of tumor-like deposits in the area surrounding the implant
Due to the damage caused by defective DePuy hip implants, revision surgery or reconstructive surgery may be needed to reconstruct bones surrounding the femur and pelvis or to replace the implant in its entirety.
DePuy Hip Implants Metallosis
Metallosis is metal poisoning caused by the release of metal ions and metal particles into the tissue surrounding the site of the implant. The release of metal fragments is usually as a result of the components of the metal-on-metal implant grating against one another. The fragments and ions may also be released into the bloodstream and travel throughout the body. Patients who get metallosis from MOM devices may experience localized swelling and pain and may also experience osteolysis and necrosis. Metal ions that have entered the bloodstream may cause systemic effects of metal poisoning such as headaches, rashes, body aches, fatigue, and other inflammation-like or flu-like symptoms.
Diagnosis of Metallosis Caused by a DePuy Hip Implant
The buildup of metallic particles in the bone, bone marrow, or blood usually results in metallosis, which has a number of damaging short-term and long-term effects. In addition, there may be a need for the removal of implant or bone/tissue and further surgeries to repair damages caused DePuy’s defective hip replacement systems. Common symptoms of metallosis include:
- Black tissue
- Tissue mass or rash
- Thigh or groin pain
- Bone deterioration
- Spontaneous dislocation
- Intense pain at the site of hip replacement
Long-term effects include:
- Lymphocytosis- increased lymphocytes that cause “sick” symptoms
- Carcinogenesis, which is the development of cancerous cells
- Chromosomal damage
- Need for additional surgeries to remove implants, infected bones, or mass tissue buildup
DePuy Bone Fracture
Bone fractures are also included in Depuy Hip Implant severe side effects. Metallosis may break down the bone tissue surrounding the site of the implant, weakening the femur and pelvis bones. These bones may consequently fracture and require reconstruction.
DePuy Implant Failure
The implant may become loose and cause a complete device failure. This may cause dislocation and misalignment of the joint and device and may as well limit the patient’s range of motion, resulting in pain and immobility. Some experts have observed that the device’s failure is mainly due to the design of the ASR cup component being too shallow.
DePuy Reconstructive Surgery and Revision Surgery
In many cases involving severe side effects caused by the ASR Hip Resurfacing System, ASR XL Acetabular System, and DePuy Pinnacle Hip Replacement System, patients must undergo revision or reconstructive surgery. Revision surgery may be necessary to remove the implant and replace it with another artificial device. Conversely, a patient may need reconstructive surgery to repair bone fractures and other issues related to the bone being affected by the implant. This may also call for the replacement of the defective DePuy implant.
These surgeries are usually more painful and more invasive the original joint replacement surgery. For this reason, patients are required to undergo longer rehabilitation and recovery and are also exposed to risks associated with surgery and recovery, including immobility, pain, inflammation, and infection. In addition, some patients have to undergo multiple surgeries to repair damages resulting from defective DePuy hip implants.
FDA Adverse Reports on ASR Hip Implants
Approved in 2005, DePuy’s ASR devices used the MOM construction and were developed based on the design of the Pinnacle device. And similar to most hip replacement devices, the FDA approved the DePuy products under the exemption process referred to as 510(k), which allowed for the implants to be introduced to the market without the stringent and costly clinical testing. In this case, the FDA assumed that no testing was needed because the devices are enough like other approved medical devices. This means that the devices were never fully tested in humans before they were actually marketed and sold for public use.
The FDA warned that MOM hip replacements were associated with high rates of premature failures compared to other types of hip implants. In 2017, the FDA finalized new regulation requiring the MOM hip manufacturers to submit a Premarket Approval application so as to market a new implant or continue marketing current devices.
Many patients with defective DePuy hip implants have had to endure painful revision and reconstructive surgeries to remove and replace their implants. And many more are expected to undergo these types of surgeries in the future. Since 2008, the FDA received at least 400 reports indicating serious issues with DePuy hip implants. According to data from the U.K., up to 50% of patients implanted with DePuy ASR hip replacement may need revision surgery in less than 6 years after the initial implantation surgery. The studies showed than 12 percent of patients with the DePuy hip resurfacing system and 13 percent of patients with the acetabular system required surgery to fix their implants. An analysis estimated a 40 percent failure rate of the ASR implant within 5 years of being fitted, although they were expected to last for 15 years. In contrast, traditional hip replacements have a 5 percent failure rate within five years.
DePuy Recall
The first failures of the Pinnacle hip implant began showing when a woman reported a severe infection and claimed metal poisons had been absorbed into her bloodstream as a result of the device’s failure. In 2007, a British doctor raised his concerns about the ASR devices showing a higher than expected early failure rate of 13 percent, or 1 in 8 patients in less than 3 years after the device was offered to the public. The recall came at the beginning of 2010 when DePuy Orthopaedics reported that the ASR Hip Implant was phased out due to a decline in sales. The company never mentioned any failure as provided by data from the Australian implant registry. By 2009, the health authority in Australia had provided warning notices to DePuy at least 7 times because of the severe side effects and as a result, the device was recalled.
Even with that, DePuy still did not issue a recall and even went further to claim that any statement mentioning a recall was false. It was not until 2010 that the company issued a warning to medical providers and patients about the high early failure rate despite evidence from the U.K. Australia, and other international sources. Similarly, despite the recall of the ASR devices and evidence of failure, it was not until 2013 when DePuy discontinued manufacturing and marketing of the Pinnacle devices. And while other countries had recalled the devices, they still remained on the U.S. market until after the manufacturing stopped. It’s worth noting that DePuy Orthopaedics never issued a recall, a move that may have allowed doctors to continue using the device until inventories were exhausted. The company has also not acknowledged any fault in the failures of the Pinnacle and ASR devices.
DePuy Hip Replacement Lawsuits
More than 7,000 Pinnacle device lawsuits and 10,000 ASR lawsuits have been filed against DePuy and Johnson & Johnson, its parent company. The first lawsuit was filed in California by Loren Kransky after he underwent the surgery in North Dakota in 2007. In addition, Kransky’s wife also filed a lawsuit for loss of consortium due to the effects of the DePuy implant. The case was settled in 2013, with an award of $8.3 million in damages. Three settlements were reached out of court in Las Vegas with DePuy agreeing to pay $200,000 to each claimant for injuries.
In the same year, the company agreed to settle more than 8,000 federal lawsuits related to the ASR devices for an estimated $2.5 billion. These cases were consolidated into multidistrict litigation in Ohio. An additional 2,000 cases remain in different states and local courts. Other federal lawsuits concerning the Pinnacle devices have been combined in the United State District Court for the Northern District of Texas.
DePuy Pinnacle Devices Bellwether Trial Verdicts
The first “bellwether” trial regarding Pinnacle hip devices was concluded in October 2014. This was a verdict for Jonson & Johnson and DePuy and was the only trial favorable to the defendant. Bellwether trials are more like sample trials meant to show the involved parties what they can expect in the full MDL. The company may decide to proceed to a full trial or settle the cases out of court depending on the outcome of the bellwether trials. In addition to the cases being settled in federal courts, many other lawsuits have been filed in local and state courts as well.
In March 2016, $500 million was awarded to five plaintiffs in the second bellwether trial. However, that award was reduced to $151 million in order to work within laws governing punitive damages in Texas. Johnson & Johnson even went a step further appealing to have the verdict crushed, claiming that the evidence provided did not sufficiently show that the Pinnacle devices were defectively designed and marketed. The company also argued that the jury knew of the $80 million settlement in 2011 and that the jury was allowed to hear evidence to show that the company bribed European doctors and paid kickbacks to individuals who conspired with Saddam Hussein.
The third trial ended in December 2016, with more than $1 billion being awarded to six California Pinnacle victims, who claimed that the devices led to injury, including bone erosion, tissue death, metal poisoning, and other negative health effects. But that judgment was also reduced to $543 million. Another $247 million was awarded to one plaintiff in November 2017. The defendant appealed all of these trial verdicts to the 5th Circuit but the motion was denied. The DePuy hip pinnacle metal-on-metal injury cases are handled in the Dallas court under a federal MDL.
Many more lawsuits may be expected. The plaintiffs argued that the companies rushed the devices to market and aggressively marketed the devices to the public with little testing. They also allege that DePuy and Johnson & Johnson misled doctors about the safety of the device assuring them that there was little risk of tissue damage and metal poisoning while deliberately concealing high failure rates. DePuy and J&J have denied these claims and said they were responsible for developing and marketing the hip devices. Plaintiffs could receive compensation for actual damages, including medical bills, pain, and suffering, monetary loss, wrongful death, etc. and for punitive damages meant to punish the company.
Plaintiffs Want the Cases to be Remanded
In February 2018, the plaintiffs filed an Omnibus Motion, asking the Dallas federal court to allow their cases to be remanded to the U.S. District Courts nationwide for individual trial dates. The plaintiffs’ proposal was for the court to conduct an orderly and efficient remand process in which the cases will be appropriately remanded to federal courts in New York, Texas, and California. The 60 cases were scheduled for 2019. The motion also requested for a staged discovery of the metal-on-poly hip revision lawsuits which are still pending in the MDL.
Status of the Lawsuits
Johnson & Johnson has begun settling claims that it knowingly sold defective hip replacement products and hid the dangers from consumers. The drug company is willing to pay more than $400 million to settle the claims. According to a December 9, 2018 court filing, a federal judge in Texas who has been overseeing the cases since 2011said that the company has settled or is in the process of settling about 3,300 of 10,000 cases. The terms of the accord were not read out to the public. J&J has agreed to resolve about a third of the Pinnacle lawsuits by paying an average of $125,000 million per case for a total payout of approximately $413 million. This marks the first settlement in the seven years that these cases have been in court. Instead of negotiating a global settlement, DePuy and Johnson & Johnson are resolving lawsuits using the same tactics as with vaginal mesh inserts. This involves settling individual attorneys’ inventories of Pinnacle hip implant cases. What’s more, the judge increased the amount of money reserved in all accords to cover attorney’s fees. To ensure that there were sufficient funds to cover those needs, the judge increased the holdbacks to 25 percent of each settlement.
Finding Help for a DePuy Hip Replacement Lawsuit Near Me
If you’re suffering issues with your ASR Hip Resurfacing System, ASR XL Acetabular System, or Pinnacle Hip Replacement System, you should know that Johnson & Johnson has a history with consumer complaints regarding their products. It’s important to seek information about possible risks and follow up with your surgeon to see if your medical problems are related to your hip implant, especially if you received the implant between 2003 and 2010.
At Consumer Alert Now, we can help you find an experienced DePuy hip replacement attorney to explain your legal options and help determine if you’re eligible for compensation for your injuries. Call us at (800) 511-0747 or fill out our online contact form to discuss your cases with a legal professional.