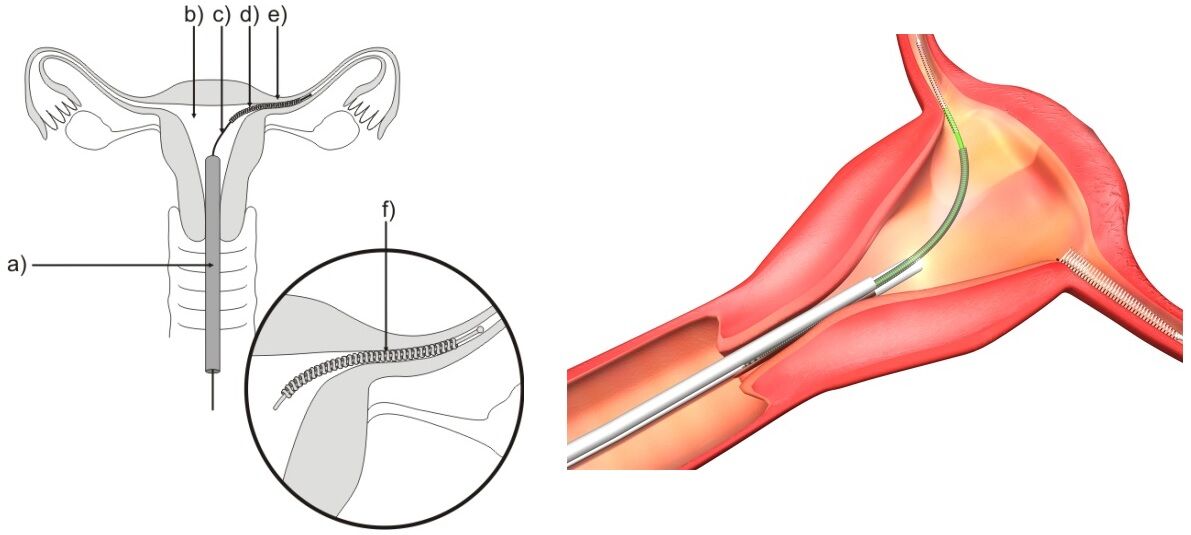
Essure is a birth control procedure approved by the FDA in 2002 that permanently prevents pregnancy. A soft, flexible insert is placed inside each of a woman’s fallopian tubes during the Essure procedure. The first three months after insertion a barrier will form around the inserts. During this period, the woman must continue with another form of birth control as a means to prevent pregnancy. The barrier that forms is made of scar tissue that will keep sperm away from the woman’s eggs so pregnancy cannot happen.
How Does Essure Work?
The Essure insert is flexible so it can bend and conform to the shape of a woman’s body and remain securely in place. These inserts are a permanent placement through a woman’s vagina and cervix, so no incisions are needed. They do not contain hormones, and the material used in their design is the same as that used in heart stents. The materials include titanium, platinum, nickel, polyester fibers, silver-tin, and stainless steel, so any patients with hypersensitivity to these may have an allergic reaction. This allergic reaction would include any woman who has a history of metal allergies.
If you suffer an allergic reaction, your symptoms could include swelling, hives, rash, and itching. There have been reports of patients developing an allergy to nickel and some of the other materials after an insertion. There has been no practical method of testing for a possible allergic reaction before having the insert placed.
During the procedure, some women experienced mild to moderate pain, and there had been cases where the doctor could not place one or both of the Essure inserts correctly. There were also cases, though rare, where the inserts broke off during placement. In these cases, the doctor had to remove the piece if possible or appropriate.
One of the confirmation tests require an x-ray, so you are exposed to very low levels of radiation as with any typical x-ray procedure. Some women have reported feeling nausea or have vomited during the process along with feeling dizzy or fainting. You may also experience pain or discomfort along with some cramping during or after the insertion.
Is Essure Right for Everyone?
Speak to your doctor about using Essure as a birth control method. Only your doctor can give you the right advice regarding the effectiveness of the insertion over other birth control options. Several areas of concern exist regarding the process, and you should know about them when thinking about using Essure:
- Essure should NOT be considered if:
- You think you might be pregnant
- You have had a tubal ligation (tubes tied)
- You have vaginal bleeding which cannot be explained
- You think or know you have cancer in your reproductive organs
- You are not one-hundred percent sure you want to end your fertility. You must be positive you are done having children as this procedure is a permanent birth control method
- You have only one fallopian tube
- You have one or both of the tubes which carry your eggs from your ovaries to your uterus is closed or obstructed
- You suffer an allergic reaction to contrast dyes used in x-ray exams
- You are unwilling to complete the Essure Confirmation Test
- You should hold-off with an Essure procedure if:
- You currently are experiencing an active gynecological infection
- If you have or had been pregnant during the last six weeks
- You are in the third or fourth week of your menstrual cycle. (There is a higher risk of being pregnant during this time of your cycle)
It is essential that you speak with your doctor when considering an Essure procedure if you are receiving therapy to suppress your immune system. If you are undergoing chemotherapy or taking corticosteroids like prednisone, that are designed to suppress the immune system, Essure may not be an effective option for you. You should also discuss any use of an IUD if this has been your previous choice for birth control.
If you are thinking about reducing the bleeding from your uterus such as the procedure which decreases your menstrual flow, you must talk to your doctor about the effectiveness of an Essure method. You should not have an Essure procedure performed the same day as this procedure. When you do have the Essure insertion, you must confirm its location through the Essure Confirmation Test.
Benefits of the Essure Insert
The benefits of the Essure insert include having no incision made for the placement. When there is no incision, there are no visible scars, and the procedure can be performed in your doctor’s office. During the process, you remain fully conscious. If this type of procedure makes you nervous or anxious, your doctor can prescribe a medication to reduce anxiety or a numbing anesthetic to reduce the risk of potential discomfort.
Essure has been considered an advancement in the female sterilization process as it performs a permanent female tubal occlusion. Older methods require incisions and blind punctures in an operating room with the use of anesthesia. Anesthesia always presents a risk, but the Essure method can achieve sterilization without any of these drawbacks.
The Essure insert is a non-hormonal birth control method for women who need this form of contraceptive, and it allows you to return to regular activity within one day or less. Sixty percent of women who have had the insert report they returned to regular activity within one day, and seventy-five percent returned within two days.
The Essure procedure takes a little over half-an-hour to complete, with more than ninety percent of women being able to have both inserts placed during the first attempt. This birth control method is more than ninety-nine percent effective at preventing pregnancies according to the data collected on the first year reliance test.
Common Side Effects or Risks with an Essure Insertion
Some women have reported experiencing mild to moderate pain, cramping, pelvic and back discomfort, and vaginal bleeding for a few days after the insertion. There have also been reports of headaches, vomiting, nausea, fainting, and dizziness accompanying the insertion of Essure. After having the procedure, it is recommended someone drive you home as there have been rare cases where the Essure insert is expelled from your body. Other reported side effects include:
- Fatigue
- Changes in your weight
- Changes in your moods including depression
- Hair loss
Long-term risks from this procedure include acute or persistent pain in varying degrees or intensity. Women who have had a history of not being able to manage pain are more likely to suffer severe pain. There have been cases where the insert was placed in the lower abdomen or pelvic area in which case it will not be a reliable form of birth control. The inserts can also perforate, or tear a hole in your uterus or fallopian tubes. An additional risk factor to keep in mind when thinking about Essure is that in clinical studies, eight percent of the women who have them placed are not able to rely on the device as a contraceptive.
If patients are hypersensitive to any of the materials used in Essure, they may experience an allergic reaction when the insert is placed. Some cases are reported where women develop an allergy to the nickel used in the inserts with symptoms typical to allergic reactions including rash, itching, swelling, and hives.
Other risks from this insert include ectopic pregnancies as no birth control method is one-hundred percent effective (based on clinical study data, the Essure insert is 99% effective). An ectopic pregnancy is one that occurs outside the uterus and is a life-threatening condition. If the Essure insert has to be removed, it will require surgery to take it out.
It should be noted that the Essure insert does not protect you from HIV or any other sexually transmitted disease. This product is only available through a prescription so talking to your doctor is required to determine whether or not it is the right choice for you.
Risk of Pregnancy with Essure
Scientific evidence shows that Essure is an effective contraceptive method when patients and the healthcare provider follow the proper instructions for its use. One study shows that after a woman uses the insert method for five years, she has only a one-percent chance of becoming pregnant.
Women are always at some form of risk when carrying a child and the risks involved if a woman becomes pregnant while using Essure are unclear. There have been reports of successful pregnancies along with healthy deliveries while a woman was using the Essure insert and there have also been reports of complicated pregnancies. The FDA received reports women were losing pregnancies after the insertions, and there was a risk for pregnancies to occur outside of the uterus with the inserts in place.
FDA Review of Essure
The FDA approved Essure in 2002 but continues to rely on a variety of postmarket surveillance data sources to ensure its continued effectiveness and safety as a medical device. They review available information about Essure and listen to experiences patients have had with the device since its approval.
During January of 2017, they received more than 11,800 medical device reports related to Essure and during 2018, more than 3,100 reports. From those reports, they were told more than eighty-five percent of the devices had to be removed from patients. The top six reasons for removals were:
- Fifty-seven percent reported the removal was due to the pain involved with the placement of the inserts
- Eleven percent reported the inserts dislocated, migrated, or expelled
- Eight-point-five percent reported genital hemorrhage
- Eight-point-five percent reported a perforation occurred
- Two percent reported the device broke
- Two percent reported a suspected allergic reaction to the metals
From these reports, most women stated they suffered from more than one reason for removing the device. There were no follow-up reports on seventy-five percent of the women who had them removed to determine if symptoms changed once the removal was completed. Data from the other twenty-nine percent included:
- Fifty-four percent reported their symptoms were resolved once the device was taken out
- Thirty percent reported symptoms were partially resolved with the removal of the device
- Sixteen percent reported their symptoms continued and they felt no improvement
The FDA discovered ten percent of those who had the device removed suffered complications directly related to the removal procedure. Some of the most common complications from the removal process include:
- Coil migration
- Fragments of the device remaining inside the patient
- The device breaking
- Procedural or post-procedural hemorrhage
- Fallopian tube or uterine perforation
Sixty percent of the device removal complaints occurred between 2003 and 2018. Forty-nine percent of these women stated they had the implants for approximately four years. The FDA continued to take concerns regarding Essure very seriously after this data was collected. During the time they began to receive concerns regarding Essure, they issued a label change request to the makers of Essure.
In 2016 it was requested by the FDA that a labeling change was necessary and they wanted an added boxed warning and patient decision checklist put into the packaging of Essure. They felt that both patients and healthcare professionals needed to be more aware of patient risks. From the information coming to them, the FDA realized that women who received the Essure insertion were not adequately informed of the product’s dangers. They wanted all women considering this form of contraceptive to have all the vital information presented before deciding on the implant.
The FDA approved the new labeling created by Bayer (product distributor) which included:
- Statement
Bayer included a statement regarding the product to advise the sale and distribution of the device be restricted to those that were willing to provide information to their patients regarding the risks and benefits of the device in a manner specified in the approved labeling given to them by Bayer.
- Discussion Checklist
The user of the device was to go over the checklist provided by Bayer and called the Patient-Doctor Discussion with any patient considering the procedure. This checklist was an acceptance of risk and informed decision acknowledgment and is part of the information booklet given to the patient. The information booklet contains essential items regarding the device, how to use the device instructions, along with safety and effectiveness outcomes the patient should know about when considering permanent birth control measures.
In 2016, the FDA also requested Bayer (distributors of Essure) to conduct a post-market study collecting data regarding Essure’s risks and benefits. They wanted data that would allow them to understand better the risks associated with Essure as compared to a laparoscopic tubal ligation. The FDA wanted information showing the rates of pelvic pain, unplanned pregnancies, or any other symptoms or side effects after an insertion along with an evaluation as to the quality of life after sterilization. They felt this data would help to understand patient complications using Essure as opposed to the women who chose tubal ligation as a means for the permanent birth control solution.
In October of 2016, the FDA sent out its final guidance to Bayer titled, “Labeling for Permanent Hysteroscopially-Placed Tubal Implants Intended for Sterilizatization” after they had reviewed public comments. This guidance was a result of feedback received from a 2015 Advisory Committee meeting and comments received through public dockets. They were being told that the packaging of Essure was not clear regarding the permanent birth control methods it imposed. The committee wanted more aggressive methods of labeling to ensure patients were well-informed of the risks involved when choosing a permanent birth control method.
In April of 2018, the FDA restricted the sale of Essure to only those healthcare professionals who would use the approved checklist titled “Patient-Doctor Discussion.” Sales would only be allowed to those healthcare providers who agreed to go over the Acceptance of Risk and Informed Decision Acknowledgment with their patients prior to insertion. When sold to these providers, the FDA felt this safety measure met their standards for a reasonable assurance of effectiveness and safety. They continue to believe that for the majority of women, the benefits of this device outweigh the risks.
A Citizen Petition Regarding Allegations related to Essure
The Office of Compliance within the Center for Devices and Radiological Health received a Citizen Petition including allegations related to Essure. The allegations included complaints surrounding clinical trial misconduct of the product stating clinical trial participant’s medical records had been altered to reflect favorable data concerning their experiences with the product.
Allegations also stated the sponsor violated terms of the Premarket Approval order and illegally manufactured and marketed Essure. Bayer was part of the FDA’s complaint investigation. They provided the FDA with case reports documenting patient experiences during their Essure clinical trials which the FDA analyzed to determine if there were discrepancies regarding the patient reported pain, comfort, and satisfaction ratings. Their findings showed that less than one percent of the cases were changed during the trials concerning bleeding, pain, pregnancy, or device movement or placement.
Though they did uncover modifications to some case reports, their analysis did not find evidence Bayer had purposefully changed the patient response to reflect a more favorable data outcome to their product, Essure.
Bayer Pulls Essure from the U.S. Markets
Bayer cited declining sales has prompted them to stop selling their permanent birth control implant, Essure. This product was removed from U.S. markets in December of 2018. Bayer's decision to stop U.S. sales comes after the FDA restricted its sales and distribution to only the women who have been well-informed before choosing the product. Bayer states the sales of Essure are no longer sustainable in the U.S. but that they stand by the device’s efficiency and safety.
Lawsuits Filed Against Bayer Due to Essure Complications
There have been thousands of lawsuits filed by women against Bayer claiming the device has caused fallopian tubes and uterine perforation along with other serious injuries. The device was developed by Conceptus, marketed by Bayer, and approved in 2002. Since then the U.S. Food and Drug Administration has received more than 30,000 reports claiming adverse side effects caused by the insert. Thousands of them are filing suit against Bayer for their injuries.
According to the complaints against Bayer, it is said the company was aware of problems with Essure but failed to make the information public. They are also accused of falsely reporting data and representing the product as safe. When Essure was developed by Conceptus and approved in 2002 by the FDA, it is reported the product was ‘fast-tracked’ and approved under specific conditions.
The conditions of the approval were that the company would provide five-year safety data after it was approved, but that Conceptus did not deliver analysis reports on time at any reporting period. Essure was then sold to Bayer in 2013. Despite the data errors and problems with adverse event problems, Bayer did not withdraw the device and instead continued to market it as a favorable risk/benefit product.
The lawsuits filed against Bayer are for their misrepresentation of consumers and not having done a fair amount of research to prove Essure’s safety. There are at least five federal Essure cases filed for negligent misrepresentation. Mass tort cases against Essure are expected to begin in late 2019.
A mass tort involves numerous plaintiffs who have suffered the same or similar injuries by an act of harm through prescription drugs, defective products, medical devices, train accidents, pollution, or a plane crash from a few defendants. The cases are heard in federal or state court and are often consolidated litigations. If you have suffered injuries from any of these situations, you should seek legal counsel to find out how you can obtain compensation.
Find an Essure Mass Tort Near Me
Consumer Alert Now can help you or someone you know who has been injured by the device known as Essure. Call us at 800-511-0747 and find out how you are allowed to file a medication/device litigation. We can help you find legal representation and discover if you can become part of the mass tort expected to be presented this year. Call today and speak to one of our professional representatives about seeking rightful compensation.