Gadolinium is a soft, bright, silvery-white metal that is both flexible and pliable and is considered a rare metal on Earth. This metal does not react in the air that is dry but will tarnish to a flaky white oxide when in moist air. Gadolinium reacts slow with water and is dissolvable in dilute acid. It will produce colorless salts.
When Gadolinium is present in compounds, it exists mostly in the trivalent state, and at room temperature, it is paramagnetic (magnetized in a magnetic field, but loses its magnetism properties when the field is removed), but when cooled it becomes ferromagnetic (retains its magnetic properties when the magnetic field is removed). The Curie point (temperature above which certain materials, such as Gadolinium, lose their permanent magnetic properties) is 17 Celcius.
What Gadolinium is Used For
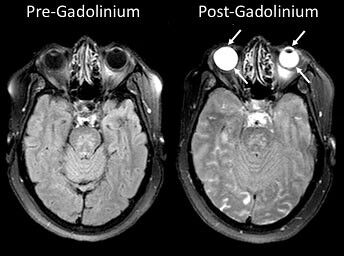
Gadolinium improves resistance to oxidation and high temperatures when used in alloys of chromium and iron. Compounds of this metal are used as green phosphors in color TV picture tubes, and because of its magnetic properties, it is also used as an intravenous radiocontrast agent for MRIs (magnetic resonance imaging).
Organic solutions of Gadolinium compounds and complexes are used as a contrast agent for MRIs to enhance images during these tests and as a phosphor, it can be used in other imaging. When the agent is injected into the body, its contrast medium improves and enhances the quality of the MRI picture.
Gadolinium (GBCA) has been used in MRIs since 1988 and used in approximately fifty percent of examinations in the United States. No other country on the globe has this high of a number. The use of GBCA as an agent in the MRI testing is the only way for gadolinium to show up in the human body, unlike other heavy metals such as lead. It is used intravenously because of its ability to improve the visualization of a person’s organs. Doctors are better able to see anomalies in blood vessels, tissues, and organs and diagnose multiple conditions.
GBCA is said to eliminate itself from the human body through urination. It is proclaimed to have a half-life of anywhere from 90 minutes to 120 minutes with a patient who has normal kidney functions.
Patient and Radiologists Concerns Over the Use of Gadolinium
In recent years, questions have begun to surface concerning the safety of gadolinium-based agents being used in MRIs. Patients and radiologists are questioning the safety of this agent in relation to the brain. In 2014 a study was released showing this agent retained and left deposits in the brain. This information along with patients making claims their health was harmed due to the use of this agent has sparked an important debate over the safety of the use of Gadolinium.
During a meeting of RSNA (Radiological Society of North America) in 2017, it became clear there was concern among many radiologists with the deposition and possible side effects of gadolinium toxicity. Radiologists are reporting their patients are showing up with side effects that can only be attributed to Gadolinium contrast agents.
Gadolinium was initially thought only to be retained in patients suffering from impaired kidneys. It resulted in a condition called Nephrogenic Systemic Fibrosis (NSF). Patients with renal insufficiency are not able to filter the substance from their bodies. A U.S. Food and Drug Administration warning label is on the contrast packaging because of this issue. It is no longer believed that only those with renal insufficiency are at risk of not being able to eliminate the contrast. Those without kidney problems, or those who have normal kidney functions, are also reporting they are retaining Gadolinium long after it should have been eliminated from their body.
Acute Side Effects from Gadolinium Contrast Agents in Children
The pediatric population has shown acute side effects from three of the commonly used Gadolinium contrast agents (GBCA). These agents are recognized as safe in clinical applications; however, there have been reports of adverse side effects from their use, including some patients who have died.
NSF (Nephrogenic Systemic Fibrosis) has gained the most attention as this is the result of devasting consequences, but there are other events linked with these contrast agents that were not as thoroughly researched in the pediatric population. Three of the contrast agents studied include:
- MultiHance (Bracco Diagnostics Inc. Princeton, NJ)
- Magnevist (Bayer HealthCare Pharmaceuticals, Wayne, NJ)
- Gadavist (Bayer HealthCare Pharmaceuticals)
The product packaging inserts for these agents provided by the manufacturers with non-published data state there is an overall rate of side effects ranging from 3.5% to 10.4 %. What is not offered is the occurrence of side effects in the pediatric population. MultiHance does report there is an overall side effect rate of 6.5% in the pediatric population; however, other agents packaging inserts do not include any specific comments related to observed rates of side effects in the pediatric population.
A prospective study using more than 130,000 doses of MultiHance in an adult population running for more than seven years, showed the overall safety of the agent. Another large multicentre study using more than 23,000 does of MultiHance showed similar low rates of adverse side effects with less than 0.7% of the adult patients experiencing common side effects of vomiting and nausea. A retrospective review of more than 158,000 patients in two separate hospitals undergoing Gadolinium contrast-enhanced MRI showed a small, but an increased rate in side effects linked to MultiHance when compared to Magnevist and Omniscan. There were no patient ages listed in this study, so it was not possible to determine the safety profiles in children.
Unique considerations and challenges exist when administering GBCAs to pediatric patients. These children are not just little adults, and cannot be considered as such when using Gadolinium contrast agents. The concern for Nephrogenic Systemic Fibrosis exits in children; however, it does appear to be lower than with adults. Even though this threat is not as prevalent in the young, the potential for risk must be taken seriously. It is recommended with pediatric patients suspected of having an abnormal renal function, be screened with a serum creatinine before an MRI scan using Gadolinium contrast agents.
Another risk aside of NSF are the concerns of a potential risk with the free gadolinium deposition. It is suggested free gadolinium deposits in the metaphyses of long bones as well as in the dentate nucleus and globus pallidus who receive multiple doses of GBCA are likely to have free gadolinium deposits.
- Definitions
Metaphyses- Narrow position of a long bone between the diaphysis and epiphysis which contains growth plates. The area or part of the bone that grows during childhood
Dentate Nucleus- Cluster of neurons or nerve cells in the central nervous system with a tooth-like edge
Globus Pallidus- Structure in the brain involved with regulating voluntary movement
Long-term effects of free gadolinium deposits are unknown but are similar to radiation exposure which makes children more vulnerable to the deposits. These deposits may be affecting the developing bone or brain in young children. Other concerns with the deposits in children are regarding the longer remaining life span of pediatric patients and if exposed as children to these agents may increase their risks for long-term complications.
Half of all MRIs performed on children contain the Gadolinium-based contrast agent to assist doctors in their visualization of normal structures and to help diagnosis pathologies such as infections or tumors. This number does not indicate that there are not certain risks involved. Parental and public awareness has increased concerning possible adverse events related to the use of the agent.
In approximately five out of 10,000 pediatric patients, non-dose related allergic-type reactions happen. Mild and adverse reactions include nausea, vomiting, dizziness, and headaches. Though these are rare, it is mandatory to administer GBCAs to children only if they are in a facility equipped to manage reactions by having physicians onsite. The Food and Drug Administration warn patients, physicians, and families to report any symptoms that may be related to contrast agents.
Acute Side Effects from Gadolinium Contrast Agents in Adults
Gadolinium-based contrast agents (GBCA) are injected into patients during an MRI (Magnetic Resonance Imaging), in CT scans (computed tomography scan), and CAT scans (computed axial tomography) to provide more explicit radiology images for a doctor to use in their patient diagnosis. Some patients are experiencing side effects after these contrast agents have been injected.
There are two types of GBCA:
- Linear
The Linear type of GBCA is chemically less stable releasing gadolinium ions. Macrocyclic GBCAs provide better protection as their molecules are bonded more securely.
- Macrocyclic
The macrocyclic are generally regarded as ‘safer’ than the linear GBCAs.
When toxic Gadolinium contained in these substances breaks away from its bonding agent, it can remain in the brain, bones, and various tissues within your body. When this occurs, there are side effects. The situation this presents can lead to Gadolinium Deposition Disease (GDD), or Gadolinium Storage Condition which is a form of Gadolinium toxicity.
Symptoms are these side effects that may appear from a few hours after your procedure to several weeks after you were injected with the GBCA. Some of the reported symptoms include:
- Thickening of soft-tissue, ligaments, and tendons
- Cognitive impairment
- Persistent headache
- The sensation of sharp pins and needles, burning or cutting
- Vomiting, nausea
- Tightness in the feet and hands
- Severe pain in your legs, arms, joints, and bones
- Brain fog or clouded mentation
- Fibrosis in skin and organs
- Skin changes
- Retention in bones
Reports received from patients living with Gadolinium toxicity have reported they have become bedridden or are now in wheelchairs as a result of their pain and loss of mobility. Other patients with less severe pain report they are experiencing loss of movement, brain fog, and changes in their skin. These symptoms are making it impossible for some patients to work, and unable to seek treatments for their symptoms as it is too expensive. At this point, there has been no cure found for these conditions.
New Class Warning for Gadolinium Retention
Gadolinium can be retained in your organs from months to years. The highest concentrations are being found in the organs, such as your liver, spleen, kidney, skins, and brain. It is also showing up as high concentration levels in bones. It varies how long the substance stays in your system depending on which organ it has concentrated in, but it appears your bones hold it the longest. Linear GBCAs are causing more retention than Macrocyclic.
When using equal doses, Gadolinium retention differs between the linear agents with Omniscan and Optimark resulting in higher retention than other linear agents Eovist, Magnevist, MultiHance. Retention is similar and lower between the macrocyclic GBCAs Dotarem, Gadavist, and ProHance.
The consequences of this retention in the brain have not been conclusive. Clinical and pathologic outcomes of GBCA administration and retention in organs and the skin has been established in patients suffering from impaired renal function. There are even rare reports of patients with normal renal function undergoing pathologic skin changes. Adverse symptoms in multiple organ systems have also been reported in patients without renal function failure that is being linked to Gadolinium retention.
Certain patients who have a normal renal function are being found to have a high risk of Gadolinium retention even though there are no clinical consequences established. The patients involved are those requiring lifetime doses, pregnant and pediatric patients as well as patients with inflammatory conditions. It is strongly recommended the retention characteristics be carefully considered before choosing GBCA for patients in these categories. GBCS imaging should be minimized in these patients.
Lawsuits Involving Gadolinium
Chuck and Gena Noriss sued manufacturers and the distributors of the contrast agent Gadolinium. It is necessary to know a little history regarding Gadolinium to understand why they are filing the lawsuit. MRIs use magnetic energy to visualize your body. Basically, large magnets are turned on and off rapidly during the test. When the magnetic is on, all the protons in our body’s water (and your body is made up mostly of water) align. When the magnet is off, the protons return to their original position. The energy they give off when they return is what creates images of our bodies.
During some cases, to make the visualization improved, a contrast agent based on Gadolinium is used. For CT scans, the solution is a slurpy size drink that has to been finished an hour before the test; for MRIs, the solution is injected into your veins. Gadolinium is being labeled toxic. In order to reduce the toxicity of Gadolinium, it is coated with another chemical used to protect your body from its effects. The two linking compounds come in two forms- spherical and linear. The spherical covers the Gadolinium more effectively. These two forms are referred to as Gadolinium-Based Contrast Agents (GBCAs).
These agents have been used in MRI studies for many years and have passed the pre-marketing under FDA testing. The FDA tests showed there were no apparent issues for eighteen years; however, after this time evidence appeared as a new condition- Nephrogenic Systemic Fluids (NFS) which showed up in patients identified with kidney failure (renal insufficiency) when exposed to GBCA. These patients suffered worsening kidney failure after exposure to GBCA.
It took some time to identify this problem due to the low number of incidences and that it did not affect all patients with kidney impairment. FDA acted fast when the identification was made by adding a warning for the use of GBCA, and the medical community’s response was also quick as they began screening patients before MRIs to look for kidney impairments. If impairments were identified, GBCA was not administered.
At the same time, NFS came to light; increasing evidence appeared showing chelating compounds were not as effective as first thought they would be. An earlier study in 1998 showed that twenty-five percent of the Gadolinium was not recovered in the patient’s urine. Another survey done in 2005 found Gadolinium showed up in some patient’s bones and yet another study in 2014 showed continued presence of Gadolinium in a patient’s brain tissue. In 2016 the American Journal of Radiology published a study titled “Gadolinium in Humans- A Family of Disorders,” and it described an extensive range of conditions characterized by the retention of Gadolinium from symptomatic patients to asymptomatic patients to patients with NFS.
In the lawsuit filed by the Norris’s, Gena claims she developed GDD (Gadolinium Deposition Disease) after experiencing three contrast-enhanced MRIs in a week. The report published by the American Journal of Radiology is part of the lawsuit filed in San Francisco. These summaries are from a few excerpts included in the study:
- A causal relationship has not been fully confirmed but is under investigation. Previous MRI gadolinium-toxicity support groups have reported symptoms considered to be consistent with known toxic effects of Gadolinium.
- GDD (Gadolinium Deposition Disease) is the name being assigned for the disease process observed in patients with a near normal or normal renal function who have developed persistent symptoms that occur hours to two months after they receive the administration of GBCA. In these cases, there was no pre-existent disease or subsequently the development of a disease of an alternate known process that would have accounted for the symptoms.
- Typical clinical symptoms included persistent headaches along with joint and bone pain. Patients were reporting clouded mentation, known as ‘brain fog,’ and there were more distinctive features similar to those seen in NFS but with lesser severity. Patients were reporting subcutaneous soft-tissue thickening as well as tendons and ligaments being painful and having a thickened appearance. There were complaints of tightness of the feet and hands, and some were experiencing extreme pain in their legs and arms most often described as a burning or cutting sensation or feeling like being stuck with sharp needles and pins.
These descriptions were consistent with Gena Norris’s complaints. What was alarming were these concerns had been brought to the attention of a patient advocacy group in 2014 formed by individuals who found one another in chat rooms as identifying with MRI-Gadolinium toxicity.
All information has now been presented to the FDA, and EMA (European Medicines Agency) and each has taken a different approach to the data. The EMA stopped the use of GBCA protected by those linear chelating agents that are not doing a good job of shielding patients from the toxic Gadolinium. They are leaving the use of Gadolinium protected by macrocyclic chelators in place. The Federal Drug Administration issued a warning about the continued use of the agents. Neither agency is declaring Gadolinium results in GDD.
- Other Countries Actions
In December 2017, the UK sent out a drug safety update concerning the use of linear GBCAs.
In January 2018, the UK stopped the production of linear GBCAs.
In March 2018, Singapore suggested the restricted use of GBCAs.
In August 2018- Korea banned 13 linear agents until the year's end and the EMA sent out a final comment which restricted the use of some linear agents and halted the use of others, while they continued to support the continued use of macrocyclic.
Chuck Norris and his wife Gena are seeking $10 million in damages for the extreme illness Gena has suffered from the effects after Gadolinium was injected into her system. Another California man filed a lawsuit in federal court claiming Gadolinium poisoned him during his MRI.
Mass Tort Litigation Overview of Gadolinium
There have been forty-two Gadolinium cases filed across the country, eight are in San Franciso Superior Court, and one in San Diego Superior Court.
MDL (multidistrict litigation is in reference to a special federal legal procedure which is supposed to hasten the process of handling complex cases) was denied in May 2018.
Several cases in California state court were remanded (returned to a lower court for reconsideration).
Currently, three District of Arizona cases are before Judge Campbell.
- General causation documents were produced
- 30B6 depositions were completed
- Defendant’s and Plaintiff’s expert reports were exchanged regarding general causation
- Daubert motions filed and hearing is set for July 3, 2019
A mass tort involves a number of plaintiffs who are filing a lawsuit against a few defendants in a federal or state court. The lawsuit is due to numerous injuries that have occurred that are similar in nature and have caused the plaintiffs severe harm. Prescription drugs are one of the top products for mass tort or civil action.
If you have been subjected to serious side effects from a prescription drug such as Gadolinium, contact legal representation to determine if you can be part of the mass tort to obtain compensation for your injuries or impaired health.
Is There Legal Help for Gadolinium Side Effects Near Me?
If you are experiencing life-altering or life-threatening side effects from taking Gadolinium contact Consumer Alert Now at 800-511-0747. It may be possible for you to file a prescription medication lawsuit, or become part of a mass tort to receive compensation for your condition. Get in touch with Consumer Alert Now and find out how we can help you through this difficult time.